Stability made simple for enterprise businesses.
Unlocking Stability for Enterprise Businesses: Simplifying Resilience with Comprehensive and Effective Solutions.
Reliable Stability Storage Service
As STAMERK, we provide an outsourcing option to meet the industry's climate-controlled storage needs.
STAMERK provides you with services for your stability studies by meeting all The International Council for Harmonization (ICH) requirements.
Specialized Process Management
Have you considered outsourcing your stability storage and sample management needs that you cannot meet for various reasons in your facility?
Why Stamerk
As STAMERK, we cooperate closely with our customers and try to meet all their needs.
We provide you with advantages by offering an alternative solution to our customers' stability study and sample storage methods.

Quality
STAMERK facility operates according to GMP and Good Distribution Practices (GDP).
Compliance with Good Manufacturing Practices (GMP) is an important criterion of pharmaceutical production to protect patient health and ensure high quality and effective medicines.

Monitoring System
Temperature and humidity conditions are continuously monitored and measurements recorded using a monitoring system compliant with FDA (21 CFR Part 11).
STAMERK monitors each air-conditioned storage area with its independent monitoring system and ensures that real-time information is instantly transmitted to the personnel.

As STAMERK, we cooperate closely with our customers and try to meet all their needs.
We provide you with advantages by offering an alternative solution to our customers' stability study and sample storage methods.
STAMERK facility operates according to GMP and Good Distribution Practices (GDP).
Compliance with Good Manufacturing Practices (GMP) is an important criterion of pharmaceutical production to protect patient health and ensure high quality and effective medicines.
Temperature and humidity conditions are continuously monitored and measurements recorded using a monitoring system compliant with FDA (21 CFR Part 11).
STAMERK monitors each air-conditioned storage area with its independent monitoring system and ensures that real-time information is instantly transmitted to the personnel.

Would you like us to be your solution partner for your crucial and costly stability storage requirements?
As STAMERK, we are ready to support you with specialized process management. You can contact us by clicking the link below.
Contact Us
Sıkça sorulan sorular
Sıkça sorulan sorulara aşağıdan ulaşabilirsiniz. Eğer sorunuzun cevabını bulamadıysanız lütfen bizimle iletişime geçin.
Which stability test studies does STAMERK stability storage service cover?
Our stability test conditions cover long-term, intermediate, and accelerated stability test studies according to the ICH Q1A(R2) guidelines. Additionally, STAMERK can provide climate control under special temperature and humidity conditions for your R&D studies upon request.
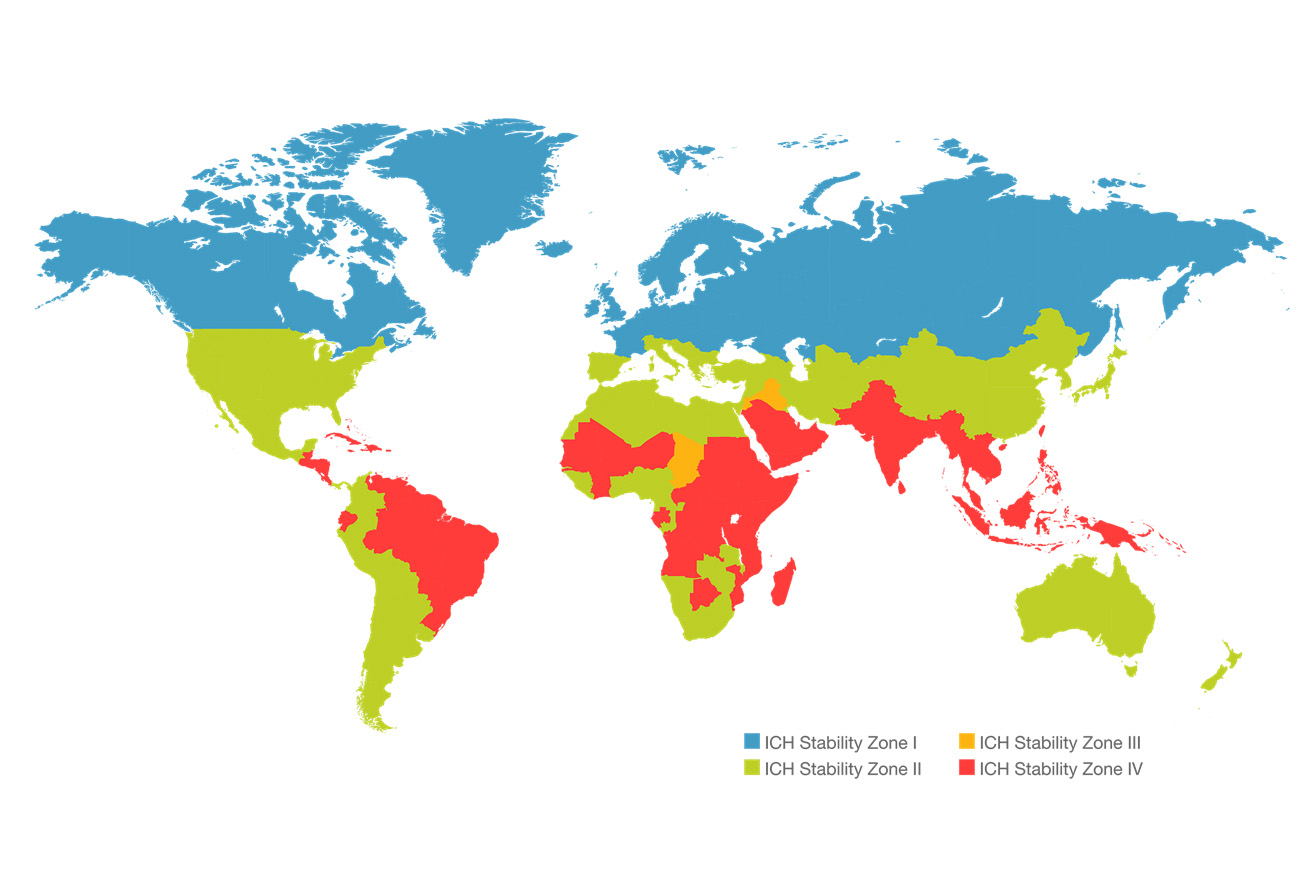
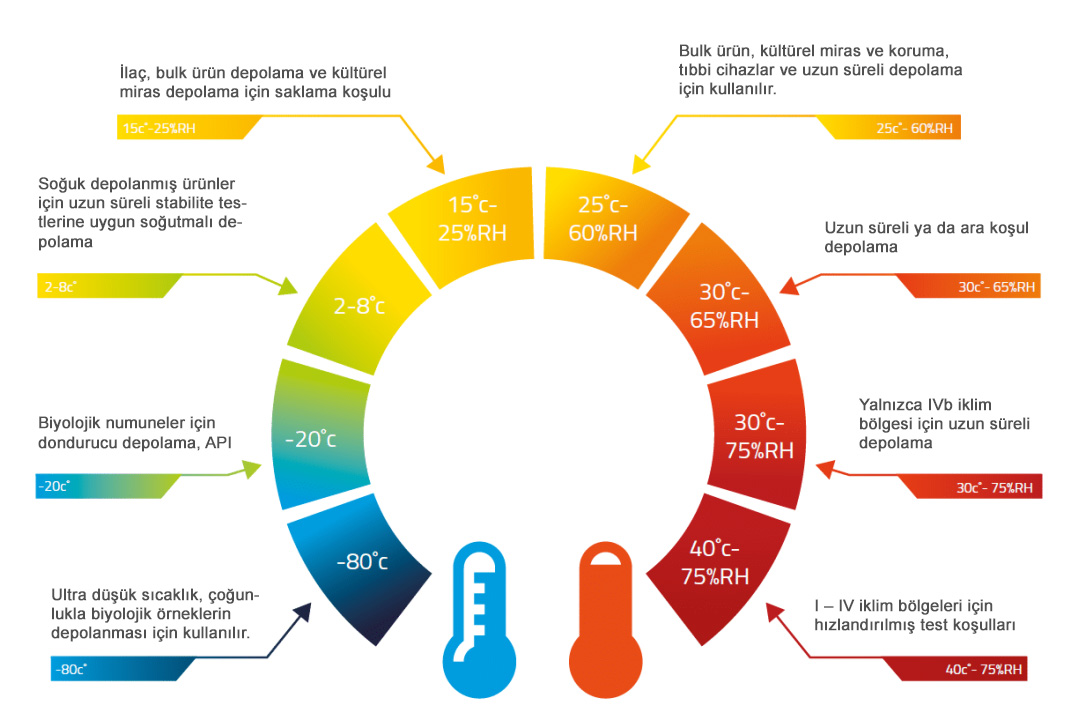
What storage conditions do STAMERK storage areas have?
- 40°C ± 2°C/75% RH ± 5% RH: Accelerated test conditions for climate zones I - IV
- 30°C ± 2°C/65% RH ± 5% RH: Long-term and intermediate test conditions for climate zones I, II, III, and IV b
- 30°C ± 2°C/75% RH ± 5% RH: Long-term test conditions for climate zone IV b only
- 25°C ± 2°C/60% RH ± 5% RH: Long-term test conditions for climate zones I and II
- 30°C ± 2°C/35% RH ± 5% RH: Long-term test conditions for climate zone III
- 5°C ± 3°C: Long-term test conditions for cold-stored products
- -20°C ± 5°C: Freezer storage for biological samples, API
- -80°C ± 10°C: Ultra-low temperature storage for biological samples
- R&D Special Conditions
How are the storage conditions of storage areas monitored?
Storage areas are monitored 24/7 with our tracking system. Measurement values are recorded and reported.
Can we rent a dedicated stability room for firm-specific stability tests?
Yes, we offer shared or dedicated space options where you can conduct all your stability tests under ICH conditions.
What advantages does a partnership with STAMERK provide?
We provide advantages for crucial aspects such as cost, maximum space, flexible capacity, and disaster and risk management by offering a reliable alternative solution for our customers' stability studies and sample storage methods.
Is a service provided for storage conditions other than ICH conditions?
Yes, you can inform us of the storage conditions you require. We work closely with our customers at STAMERK.
How does STAMERK contribute to cultural heritage preservation?
Some items in private or museum collections require storage under specific climate conditions. We provide suitable climate conditions for the preservation of cultural heritage collections at the STAMERK facility, serving as an external resource option for reliable storage spaces in case of natural or human-made disasters. We offer primary or emergency storage solutions.
What products can we store at STAMERK facilities, aside from stability drug storage?
- API, vaccines, blood and plasma, explant device storage (e.g., hip prostheses), pathology samples, bone, DNA/RNA, liquids and urine, agricultural and plant samples, serum, and other biological materials.
- Documents, books, manuscripts, and catalogs, drawings and engravings, historical records, videos, microfilms, film archives, pictures and fine arts, musical instruments, archaeological artifacts.